一、 Introduction to certification
The ISO13485 certification standard is the basic criterion for the production and quality management of medical devices. It is applicable to the key processes that affect the quality of the finished product during the whole production process of medical device preparations. It can strengthen the management of medical devices, strengthen the quality control of the enterprise, and ensure the personal safety of patients; providing users with stable quality products can improve and improve the management level of the enterprise and increase the reputation of the enterprise; improve and ensure the quality level of the product, so that the enterprise Obtain greater economic benefits; help eliminate trade barriers and obtain a permit to enter the international market; help enhance the competitiveness of products and increase their market share. The release of the 2016 version of the ISO13485 certification standard provides an important prerequisite and opportunity to promote the quality management of my country's medical device production, and it also puts forward higher requirements. Drawing lessons from the experience of the US FDA, according to the practices of the US and some European countries, promote the work of medical device production quality management standards.
The implementation of the 2016 version of ISO13485 is conducive to the management level and product quality of medical device manufacturers in my country to a new level, is conducive to ensuring the safety and effectiveness of medical devices, is conducive to the deepening of medical device supervision and management, and is conducive to the development of medical device quality certification. Development is conducive to the healthy and rapid development of my country's medical device industry.
After the ISO13485:2016 standard was released, the State Food and Drug Administration was transformed into the industry standard YY/T0287—2017 "Requirements for the Medical Device Quality Management System for Regulations" on September 17, 2003 in accordance with the principle of equivalent adoption. Medical devices Manufacturers, operating companies, medical device regulatory agencies, medical device certification agencies, testing agencies, medical device users and related units should all learn and implement the ISO13485:2016 standard to effectively improve the overall level of my country's medical device industry.
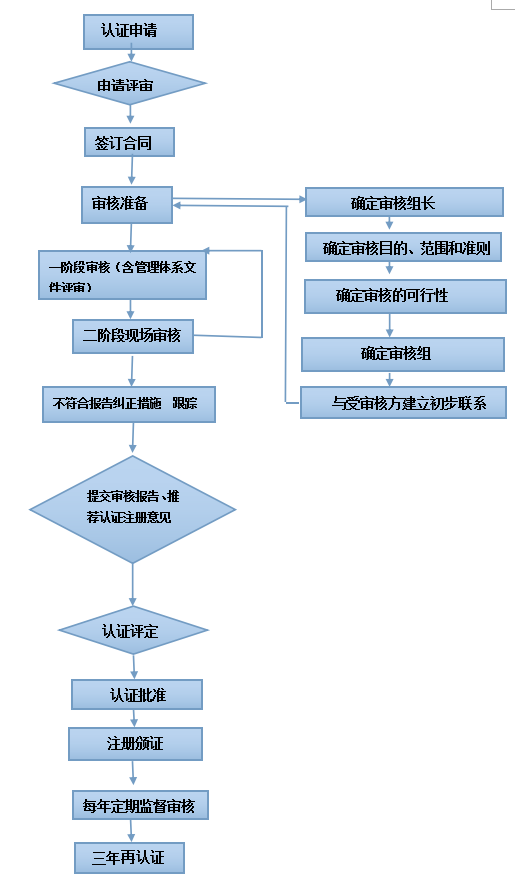
二、 Certification process